In recent years, there have been countless articles, discussions and reports on the miracle material called "graphene". It is one of the hardest and most resilient materials in the world and has been on everyone's lips since the Nobel Prize in 2010 at the latest. Because of its many advantages (e.g. very flexible, almost transparent, 100-300 times stronger than steel, very good heat conductor, etc.), it has enormous economic potential and could be used in the future for the production of solar cells, displays and microchips.
Phosphorene versus graphene
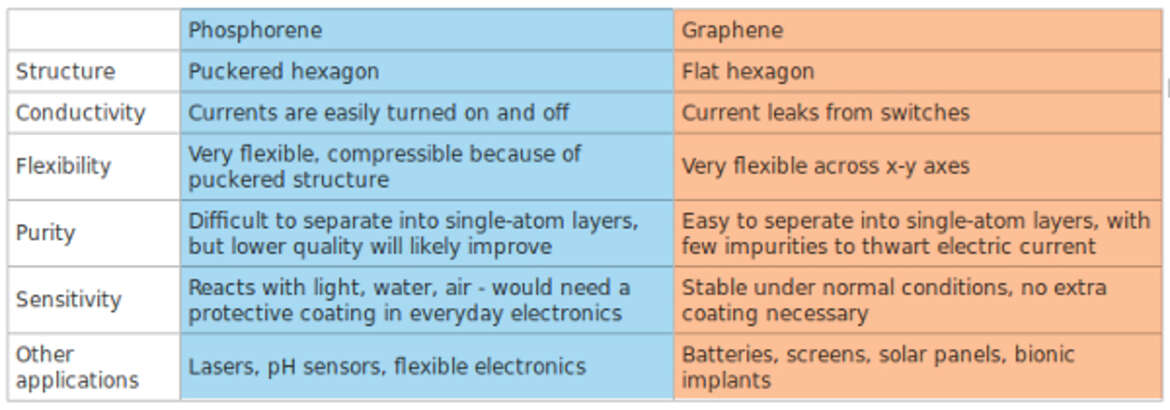
For some time now, however, it has looked as if graphene has faced competition from the non-toxic, black phosphorus (phosphorene). Which, like graphene, has a two-dimensional atomic layer. However, it has a much larger bandgap than graphene, making it a more promising candidate for nanotransistors. In addition, scientific studies by Trinity College Dublin under the direction of Jonathan Coleman now also confirm the suitability of black phosphorus for mass production.
Low-cost manufacturing process
Black phosphorus is usually formed from white phosphorus under high pressure (12,000 bar) and elevated temperature (200 °C). However, there has recently been a newly developed method of synthesizing black arsenic-phosphorus without high pressure. Which is cheaper due to the less energy required. The method was developed in cooperation between the Technical University of Munich (TUM) and the University of Regensburg, as well as the American universities of Southern California (USC) and Yale.
If you would like to learn more about the two research results mentioned here, you can find more information at the URLs mentioned in our reference. In any case, we can be curious to see what innovative solutions will be presented to us in the next few years with the new graphene competitor.